
Back أكسيد المنغنيز الثنائي والثلاثي Arabic منقنز (II, III) اوکسید AZB Mangan(II,III)-oksid BS Oxid manganato-manganitý Czech Mangan(II,III)-oxid German منگنز (II, III) اکسید Persian Oxyde de manganèse(II,III) French Tetrossido di trimanganese Italian Манган(II,III) оксид Macedonian Óxido de manganês(II,III) Portuguese
| |
| Names | |
|---|---|
| IUPAC name
manganese(II) dimanganese(III) oxide
| |
| Other names
Manganese tetroxide; Manganese oxide, Manganomanganic oxide, Trimanganese tetraoxide, Trimanganese tetroxide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.013.879 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Mn3O4 MnO·Mn2O3 | |
| Molar mass | 228.812 g/mol |
| Appearance | brownish-black powder[1] |
| Density | 4.86 g/cm3 |
| Melting point | 1,567 °C (2,853 °F; 1,840 K) |
| Boiling point | 2,847 °C (5,157 °F; 3,120 K) |
| insoluble | |
| Solubility | soluble in HCl |
| +12,400·10−6 cm3/mol | |
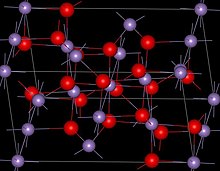
| Structure | |
| Spinel (tetragonal), tI28 | |
| I41/amd, No. 141 | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
C 5 mg/m3[1] |
REL (Recommended)
|
None established[1] |
IDLH (Immediate danger)
|
N.D.[1] |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
149 J·mol−1·K−1[2] |
Std enthalpy of
formation (ΔfH⦵298) |
−1387 kJ·mol−1[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.